Nonte Technologies Inc.
Powering the Economics of Discovery
Streamline Your Path to Regulatory Approval and Faster Returns.
Used by leading research organizations
Introducing Nonte
Building the foundation for a future where no breakthrough is lost to broken data.
1
A System for Translational Complexity
Purpose-built to address the unique data and workflow challenges in translational research environments.
2
Assured Regulatory Confidence
Ensures data integrity, audit readiness, and compliance alignment across every stage of the approval process.
3
Optimized for Research ROI
Reduces costly rework, accelerates timelines, and maximizes the return on every research investment.
Our Platform, Your Foundation
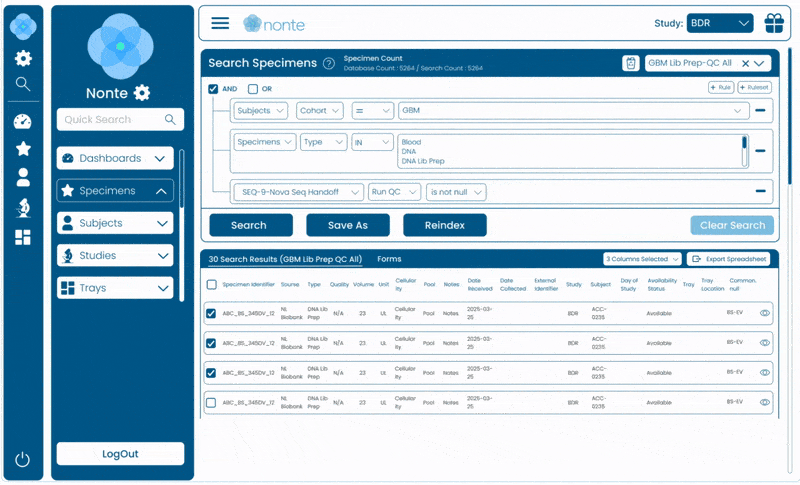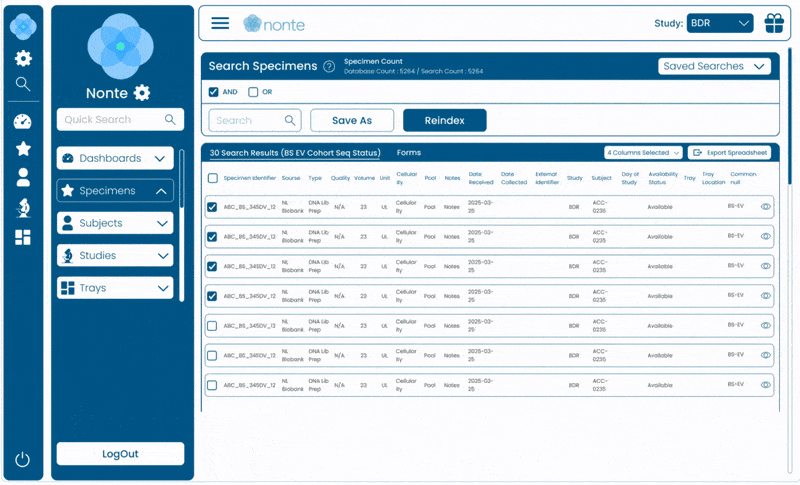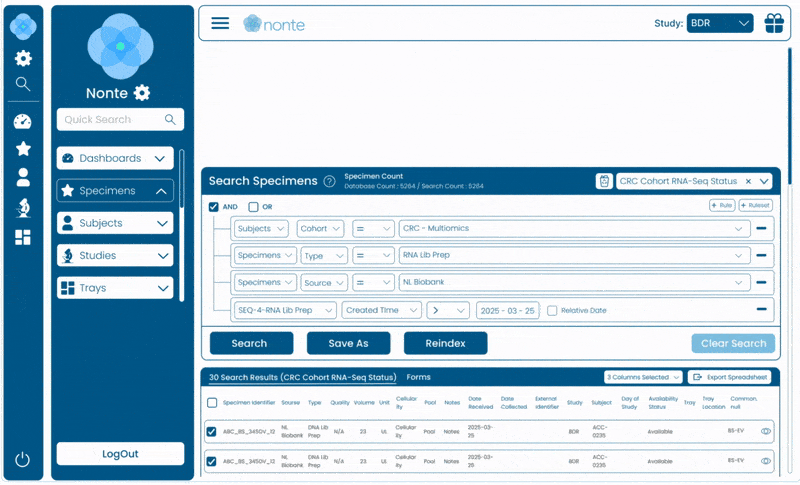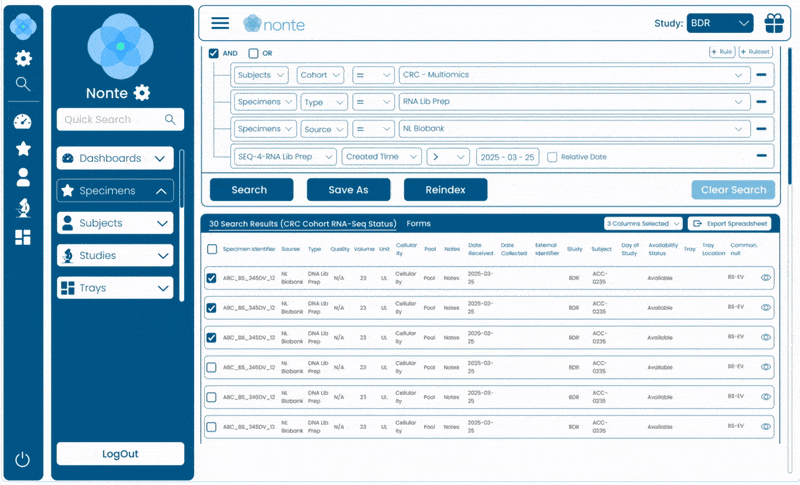
Complete Data Traceability
Tracks every sample, event, and decision from study design through regulatory submission.
Seamless System Integration
Connects effortlessly with existing lab tools, databases, and study planning environments without disruption.
Real-Time Oversight and Control
Empowers teams and funders with live study visibility, performance metrics, and audit-ready confidence.
Delivering on Our Promise

1
Adaptive Study Configuration
Adaptive Study Configuration
2
Structured Data Architecture
Metadata-driven design ensures consistency, compatibility, and validation across every dataset and workflow.
3
Dedicated Support Partnership
Every client is assigned a study-specific support lead for proactive help and seamless rollouts.
Case Studies
Stories from our Clients
Frequently asked questions
Got questions? You’re not alone, here are a few our team hears all the time.
What is Nonte, in simple terms?
Nonte is a cloud-based platform designed to enhance data traceability and oversight in translational research. It connects study workflows, data points, and documentation into one audit-ready environment, ensuring that every aspect of your research is accurately tracked and easily accessible.
Will it replace my current systems?
No, Nonte is designed to integrate seamlessly with the tools you already use, such as REDCap, EDCs, and lab systems. This means you can enhance your existing workflows without the need to replace or overhaul your current systems. But if you are in the market for a new system, Nonte can replace current systems end to end if needed.
How hard is it to implement?
Nonte is quick to configure and doesn’t require major IT involvement. Most teams can be up and running in days, not months, allowing you to start improving your data management processes almost immediately.
What will this cost—and what do I save?
Pricing for Nonte is scalable based on study size and usage, designed to accommodate research budgets. By reducing errors, delays, and compliance issues, Nonte always saves more than it costs, providing a significant return on investment.
Who is already using it?
Nonte is being implemented by academic institutions, accelerators, and translational research programs in Canada, USA and the UK, including University of Houston, Atlantic Cancer Consortium, Houston Methodist, Aston University and many others. Our user base is growing rapidly, reflecting the platform’s effectiveness and adaptability.
Is this just for regulated clinical trials, or earlier too?
Nonte supports the entire translational journey from preclinical studies to regulatory submission and impact studies. It’s especially valuable in early-phase research, where traceability and structure are often lacking, ensuring that your data is robust from the outset.
How secure is my data?
Your data is encrypted, securely stored, and always under your control. Nonte is built with strict data governance and compliance in mind, ensuring that your information remains confidential and protected.